For cosmetically conscious patients with actinic keratosis lesions on the face or scalp
A MODERN FACIAL FIELD THERAPY WITH MINIMAL DOWNTIME1-3
view safety dataSeverity of AK treatment-related skin reactions are among the many factors that contribute to nonadherence with topical therapy and may negatively impact patient compliance and satisfaction.4,5
Visual assessment of local skin reactions
SEE PHOTOS OF LOCAL SKIN REACTIONS FROM BASELINE THROUGH DAY 576*
The mean local skin reactions composite score after treatment with KLISYRI® peaked on Day 8, rapidly decreased by Day 15, and resolved by Day 292
FACE: SEVERE reactions*
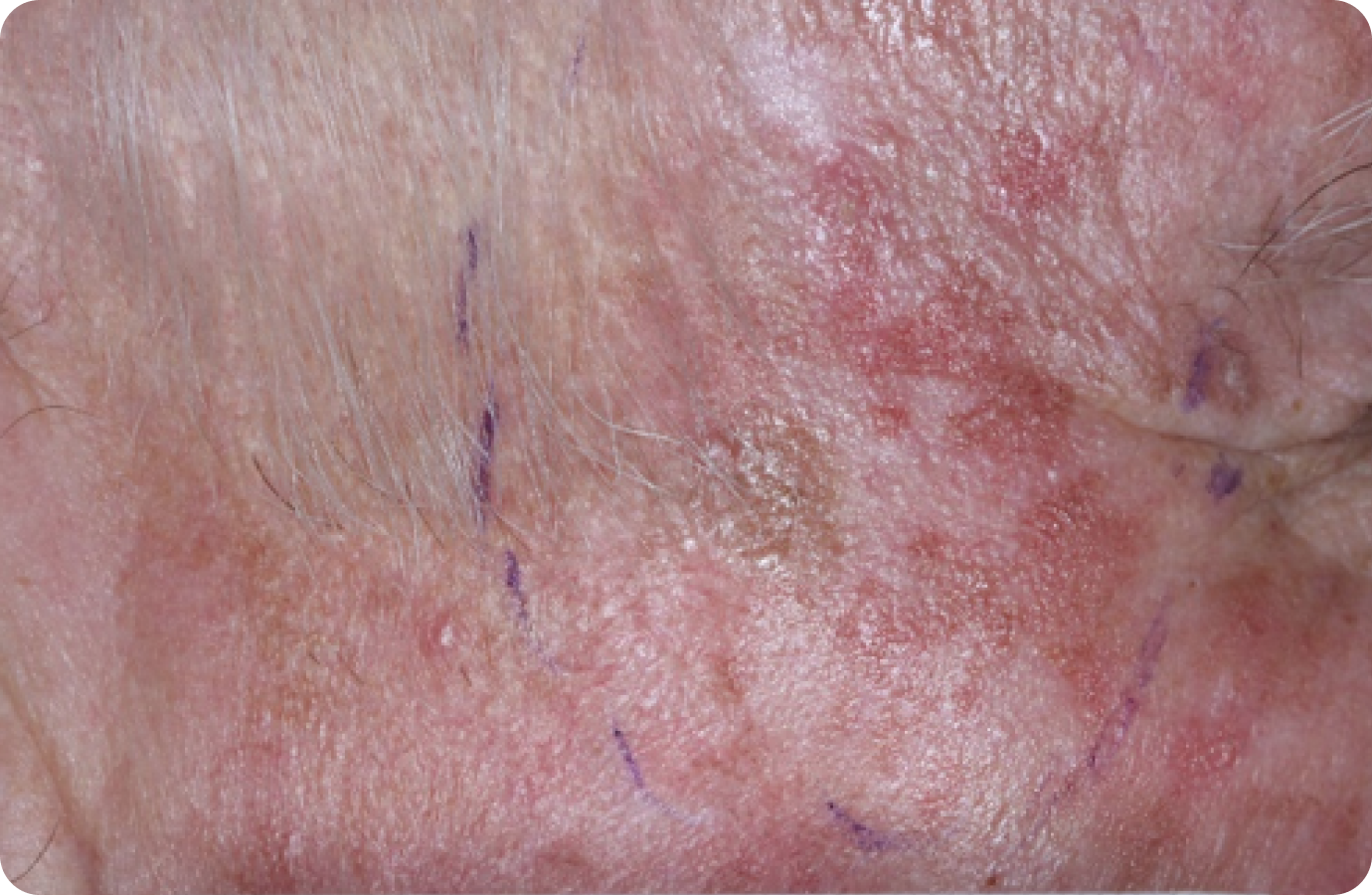
Day 1-Baseline
69 years of age/white male/Day 8/severe erythema and flaking/moderate crusting/mild swelling

Day 1
Baseline
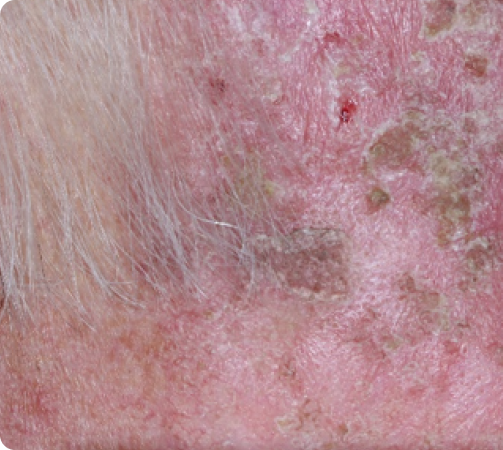
Day 8
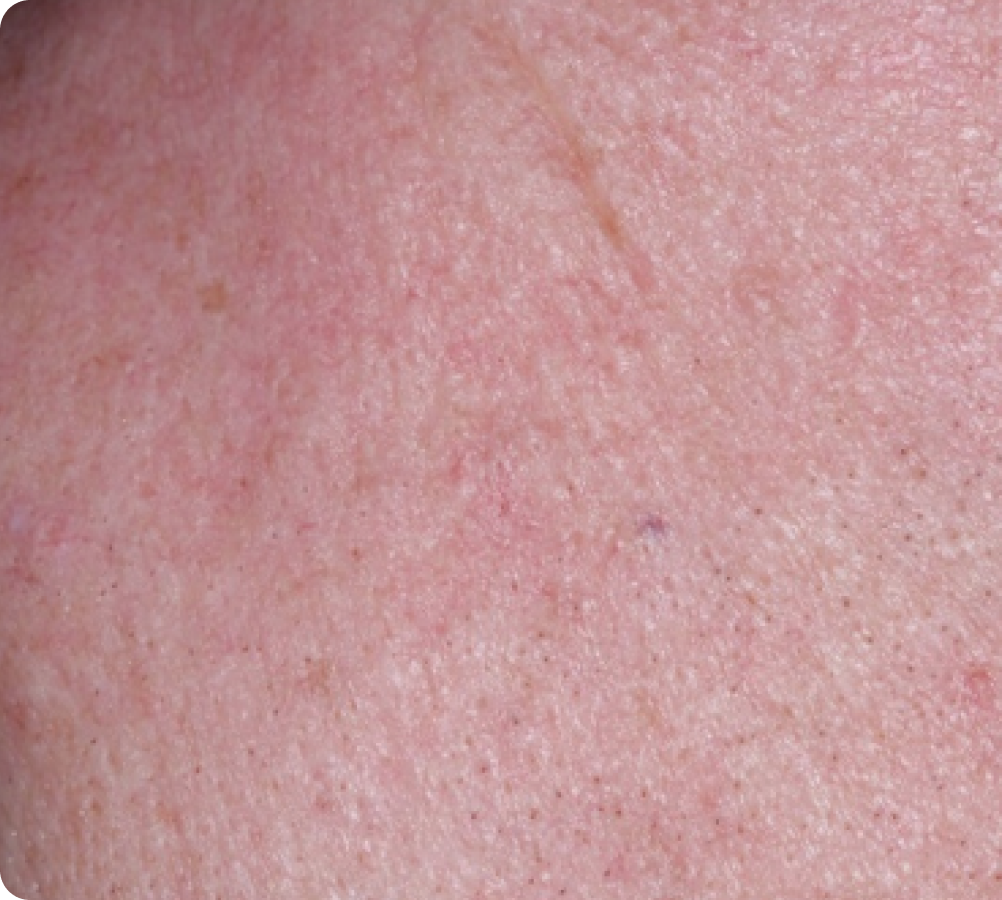
Peak local skin reactions
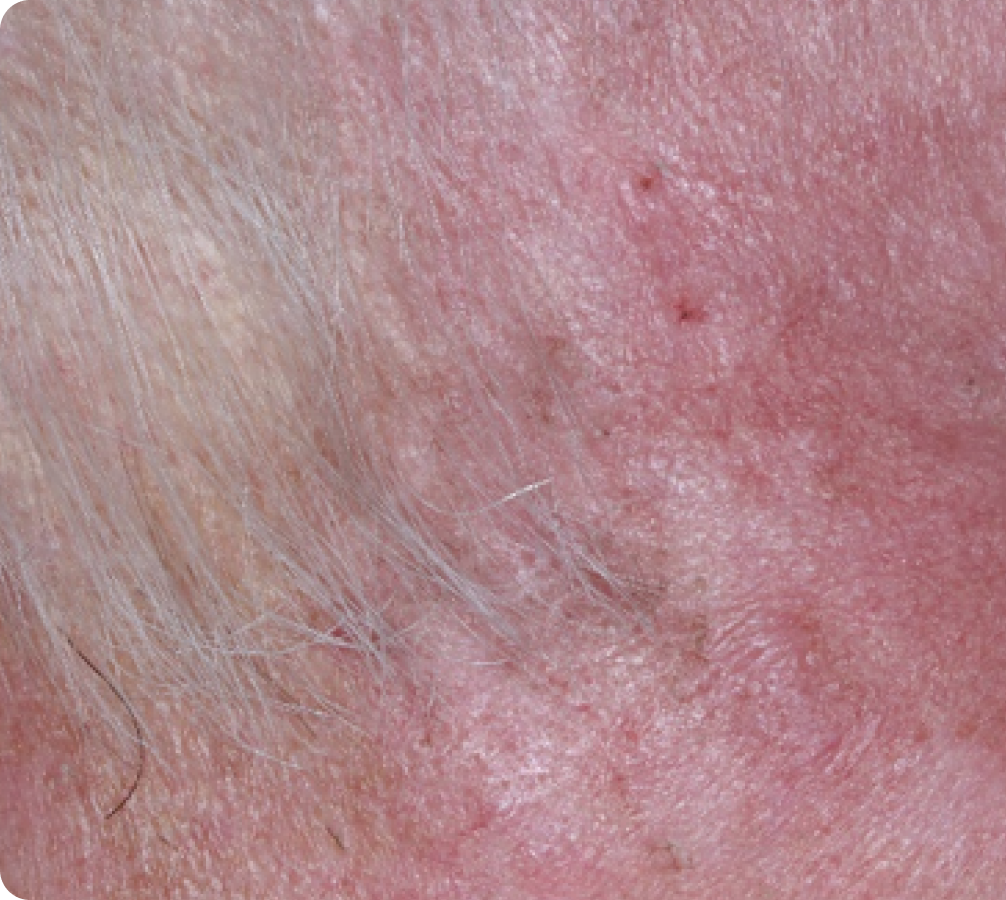
Day 15
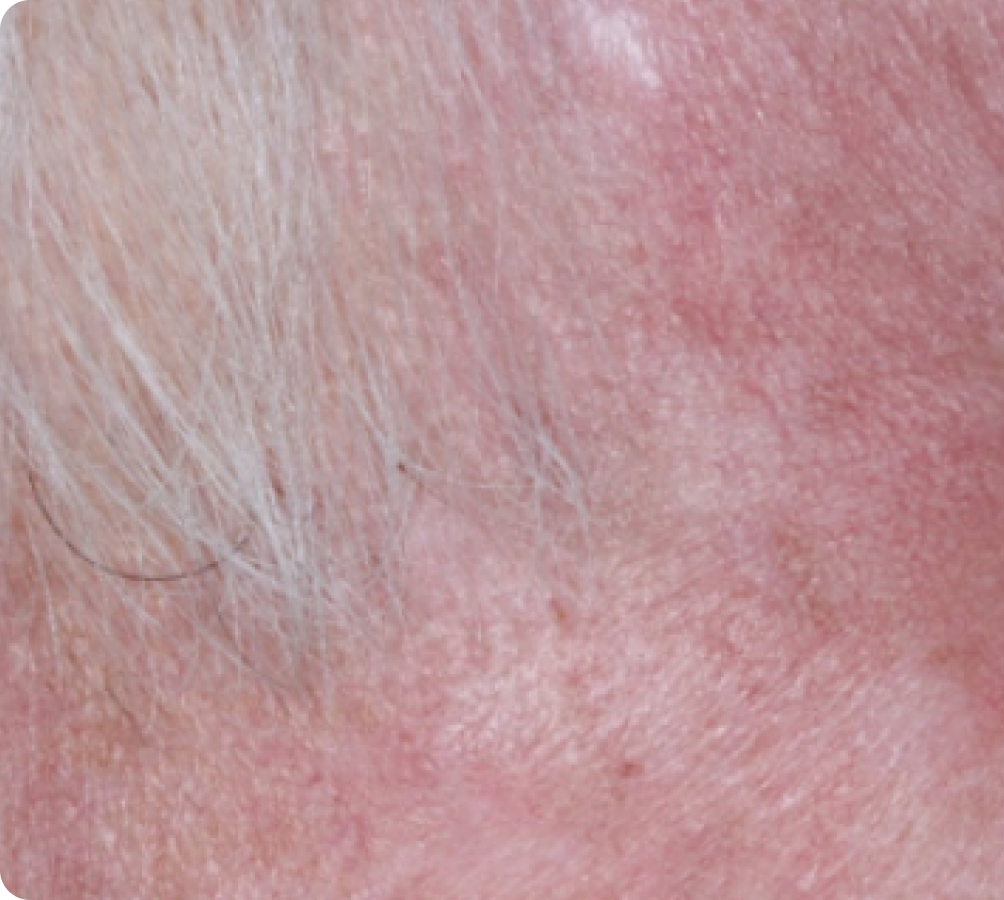
Day 29
Back to baseline
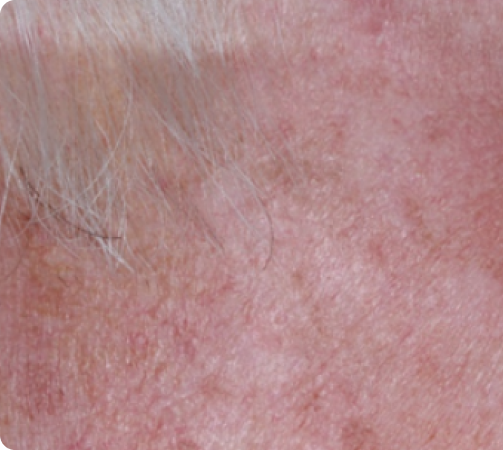
Day 57
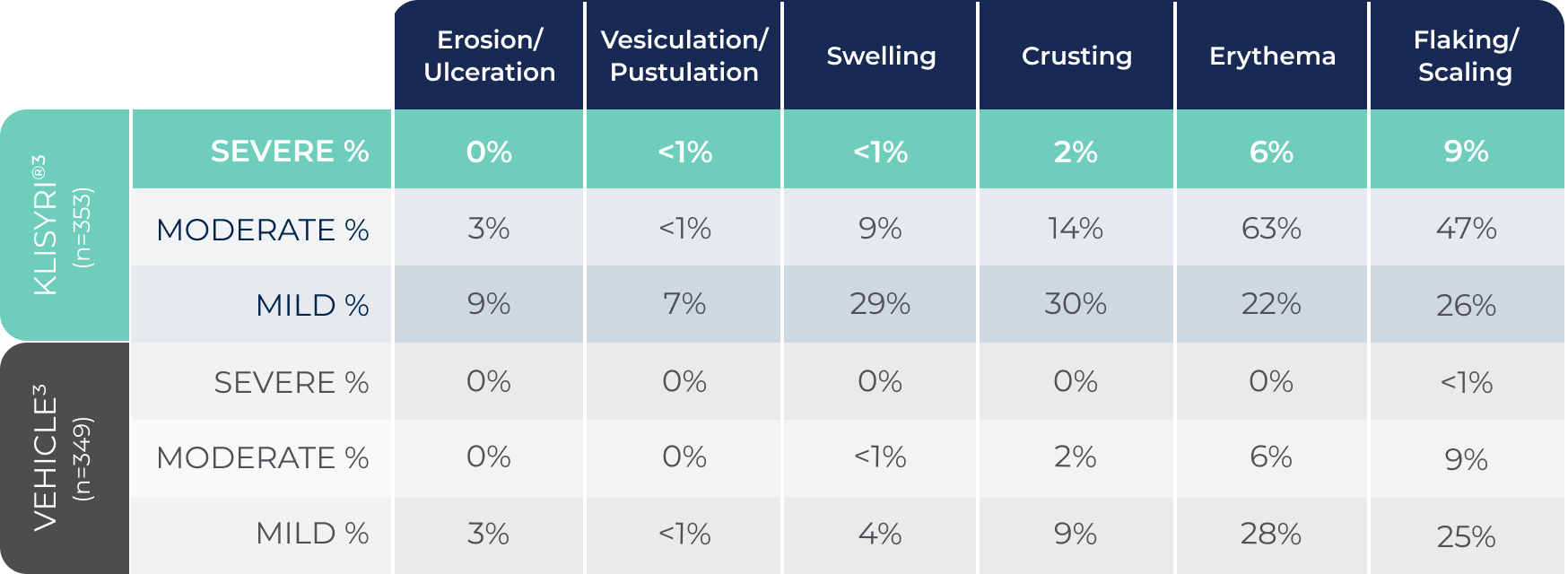
*Most local skin reactions were mild to moderate. The rates of severe local skin reactions were:
Erosion/ulceration: 0%; Vesiculation/pustulation: <1%; Swelling: <1%; Crusting: 2%; Erythema: 6%; Flaking/scaling: 9%.1
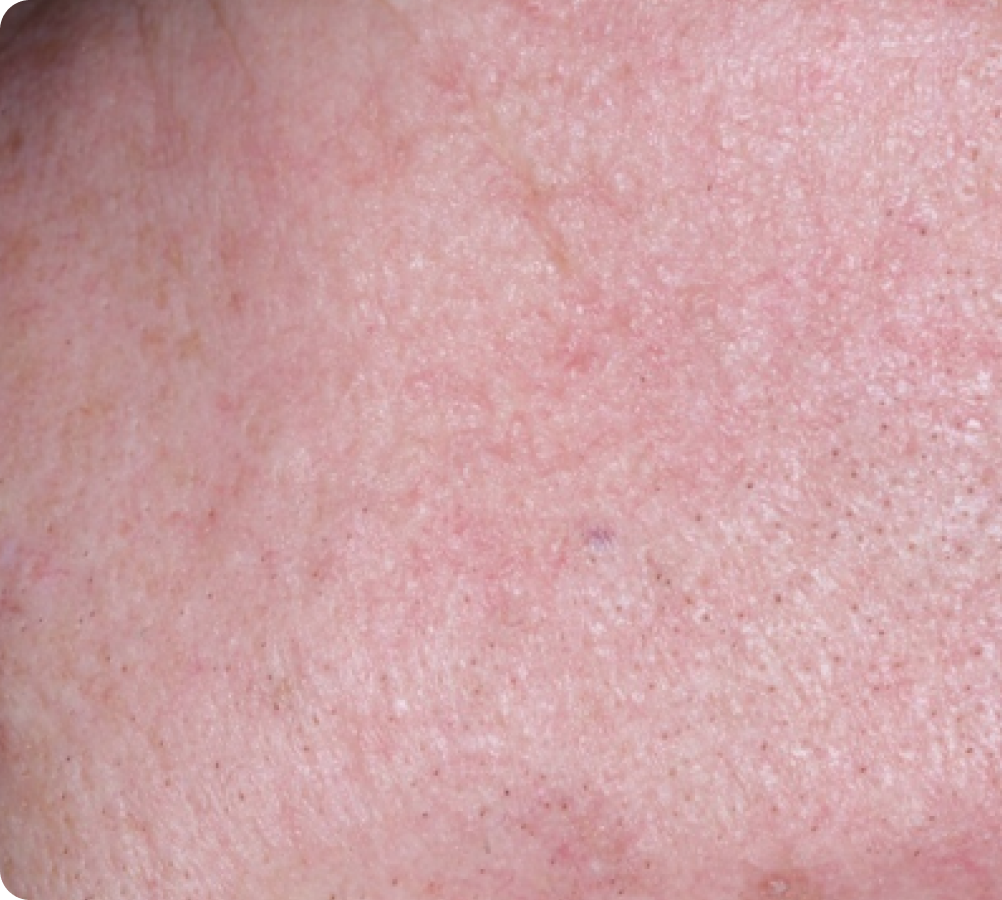
FACE: moderate reactions
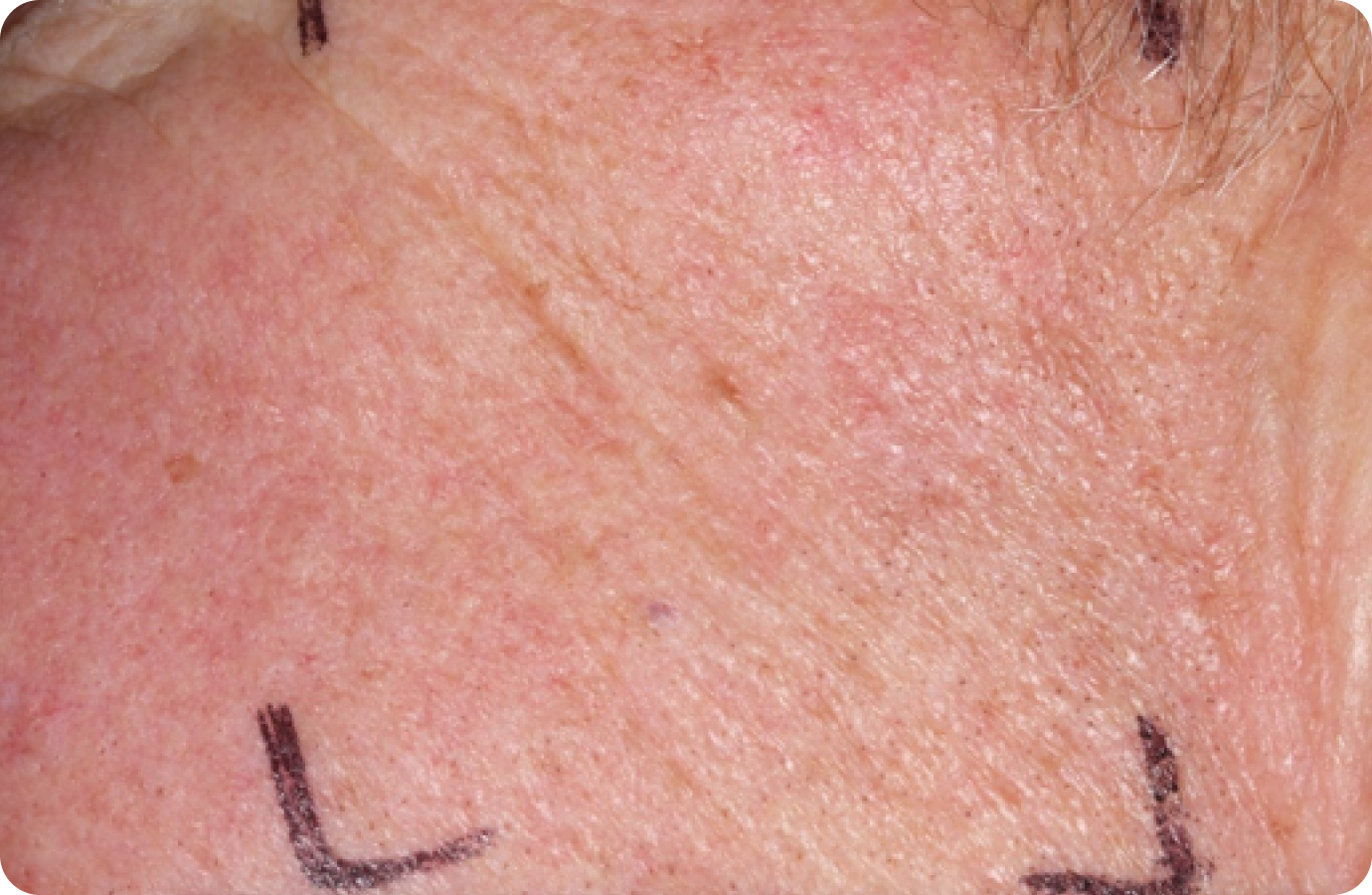
Day 1-Baseline
56 years of age/white female/Day 8/moderate erythema/moderate flaking/mild crusting

Day 1
Baseline
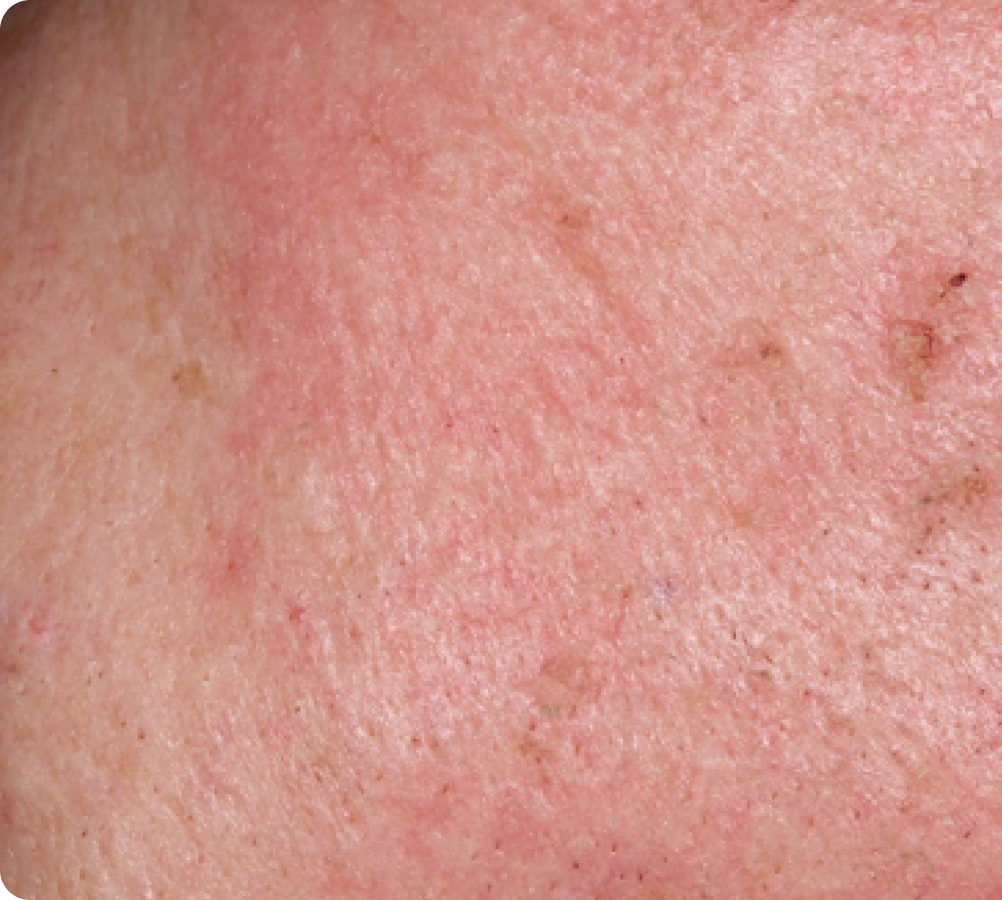
Day 8
Peak local skin reactions
Day 15
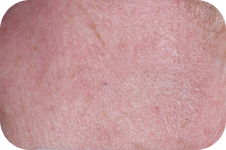
Day 29
Back to baseline
Day 57
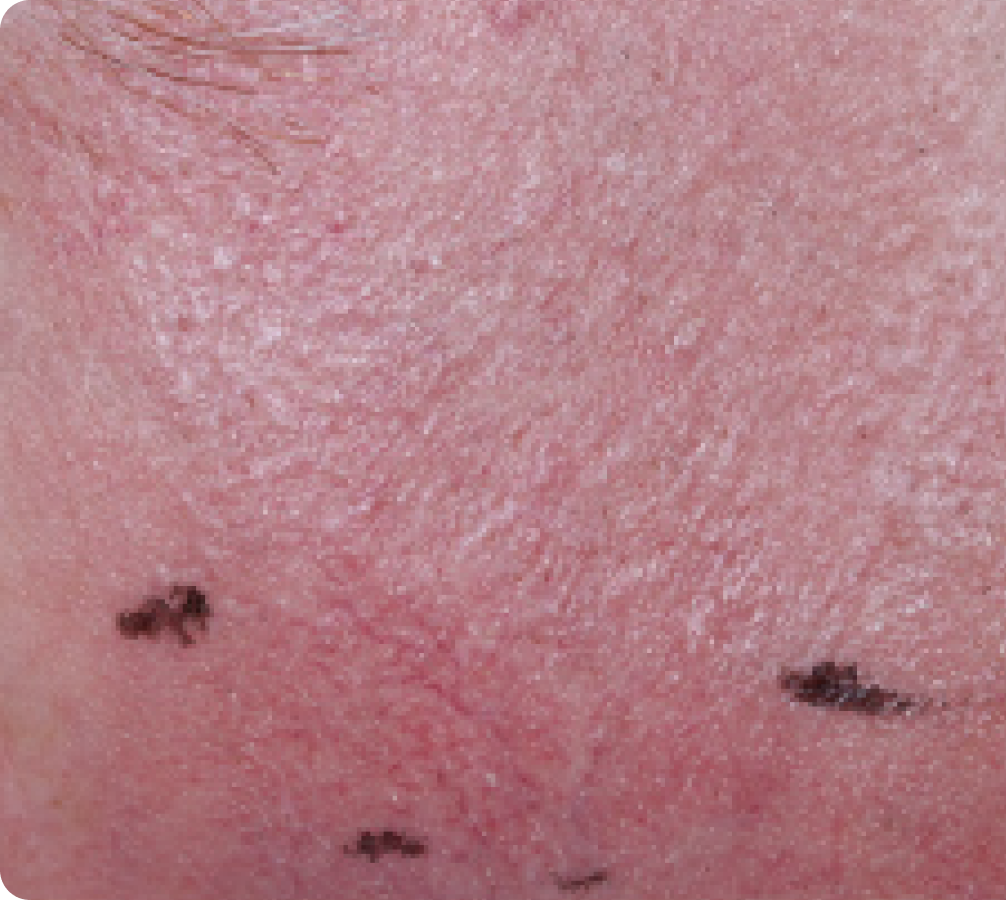
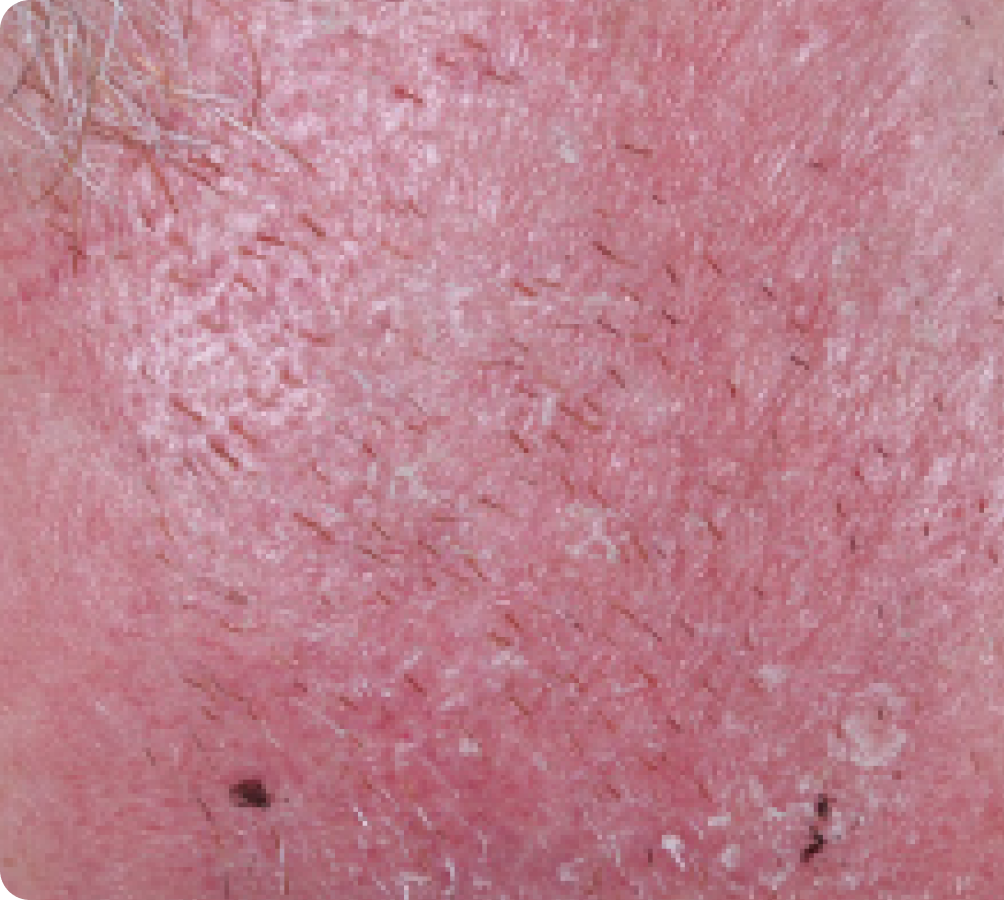
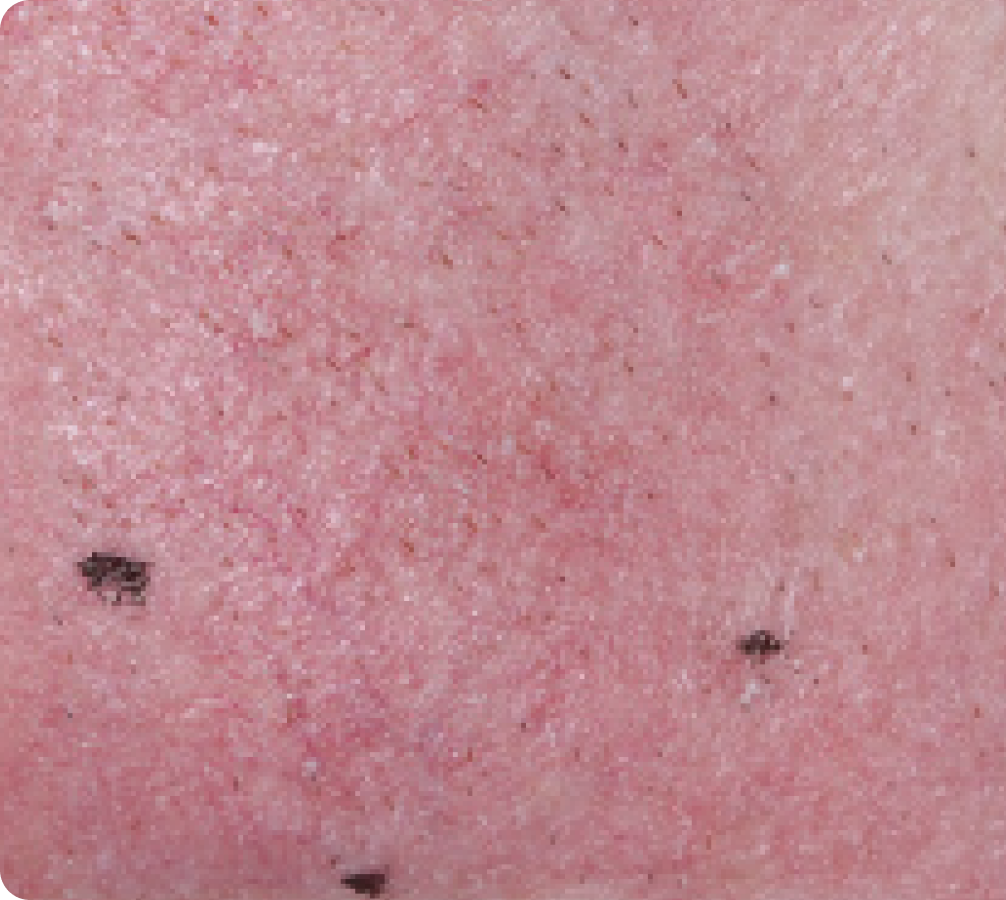
FACE: mild reactions
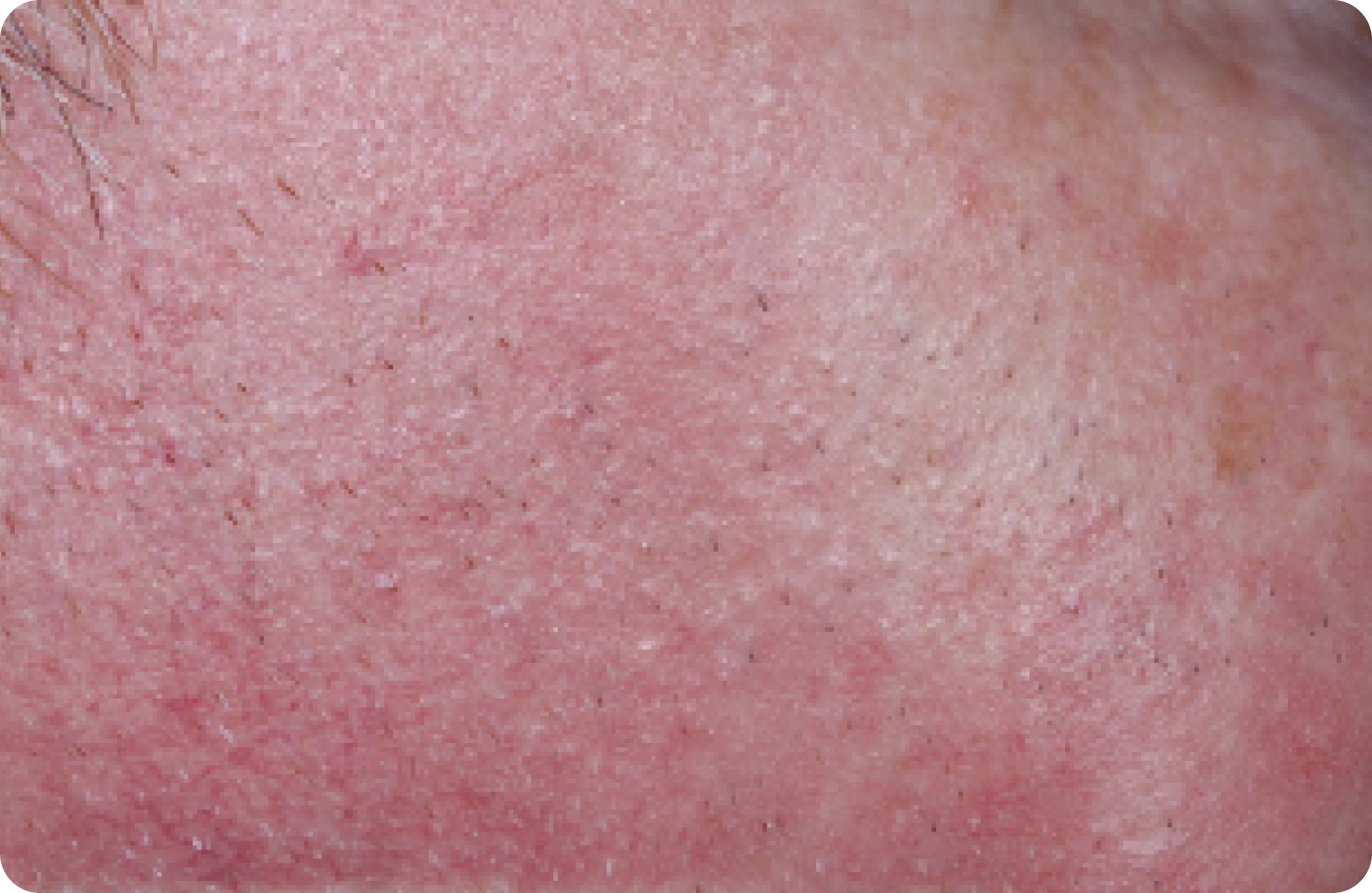
Day 1-Baseline
58 years of age/white male/Day 8/mild erythema/mild flaking
Actual clinical trial subject. Results may vary.

Day 1
Baseline
Day 8
Peak local skin reactions
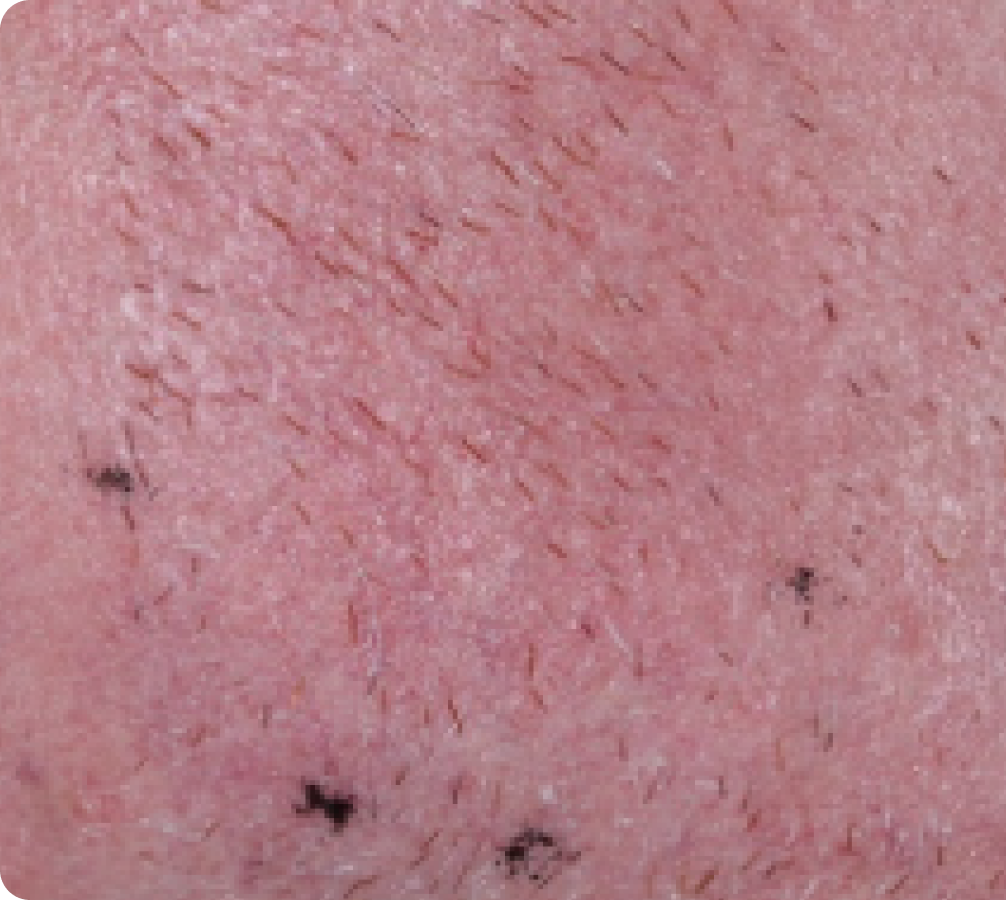
Day 15
Day 29
Back to baseline
Day 57
KLISYRI® has an established safety and tolerability profile as demonstrated in the largest pivotal clinical trial of a topical AK treatment1,7
MOST LOCAL SKIN REACTIONS WERE MILD TO MODERATE1
Local skin reactions peaked by Day 8
By Day 29, all signs were at or below their baselines in both incidence and severity2,8,9

Hear board-certified Texas dermatologist, Tricia Brown, MD, FAAD, discuss her own positive facial field therapy experience with KLISYRI®
Watch videoProposed mechanism of action
KLISYRI® is a microtubule inhibitor indicated for the topical treatment of actinic keratosis of the face or scalp1
The mechanism of action of KLISYRI® for the topical treatment of actinic keratosis is unknown1
See MICROTUBULE INHIBITION in action
WATCH VIDEO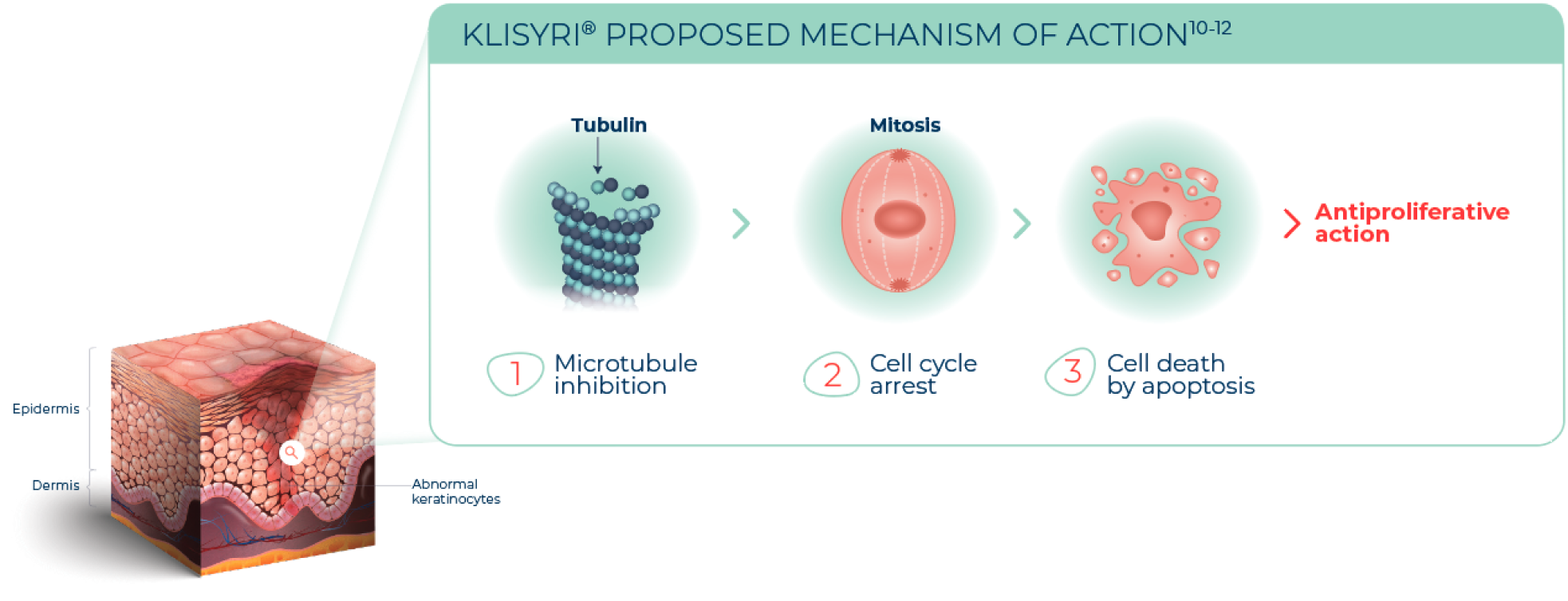
SEE MICROTUBULE INHIBITION IN ACTION
WATCH VIDEOAdverse reactions occurring in
≥2% of subjects in 2 controlled clinical trials (pooled safety populations)1
In an additional multicenter, open label safety study of 105 subjects where KLISYRI® was applied to a treatment field of 100 cm2 on the face or scalp, the results were comparable to the safety profile established by the controlled studies in a 25 cm2 treatment area.1
*Application site pain includes pain, tenderness, stinging, and burning sensation at the application site.1
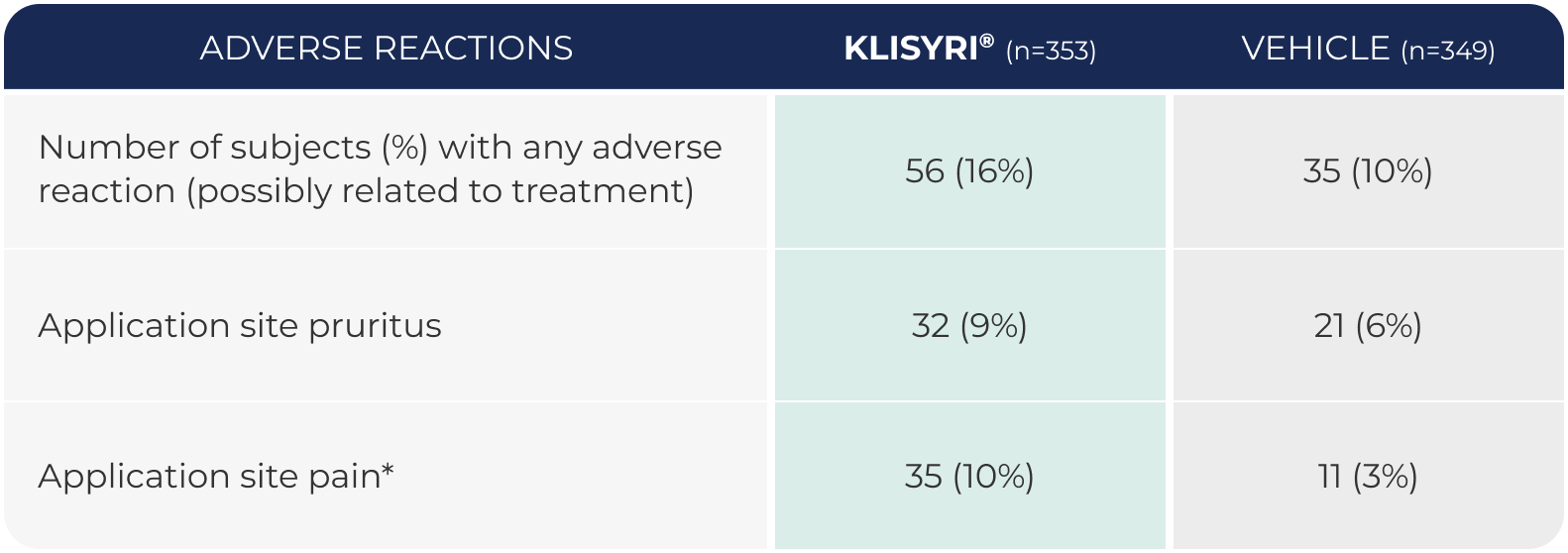
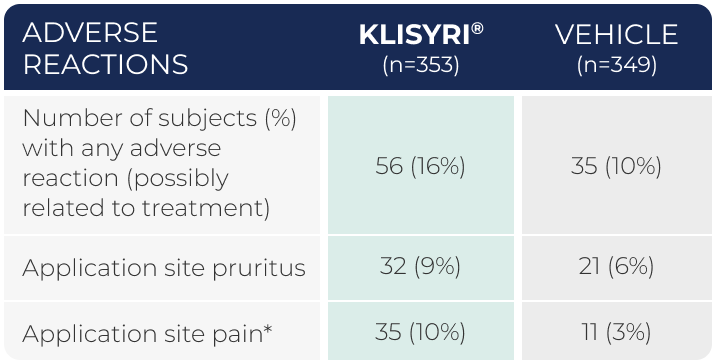
*Application site pain includes pain, tenderness, stinging, and burning sensation at the application site.1
No phototoxicity or photoallergic reactions
A modern, 5-day facial field therapy you can prescribe any time of year1
Many topical AK treatments carry a photosensitivity warning.1,13-15
No phototoxic or photoallergic skin reactions were reported among healthy subjects using KLISYRI® in clinical studies.1
No phototoxic skin reactions1
(31 subjects)
No photoallergic skin reactions1
(64 subjects)
No contact sensitization1
(261 subjects)
Discover why 5-day AK field therapy is different
Learn more about 5-DAY DOSINGIn a real-world evidence study, more than
75% of patients received KLISYRI® (tirbanibulin) during the summer months
In a real-world evidence study, more than 75% of patients received KLISYRI® (tirbanibulin) during the summer months
IMPORTANT SAFETY INFORMATION
INDICATION
KLISYRI is a microtubule inhibitor indicated for the topical field treatment of actinic keratosis on the face or scalp.
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Ophthalmic Adverse Reactions
KLISYRI may cause eye irritation. Avoid transfer of the drug into the eyes and to the periocular area during and after application. Wash hands immediately after application. If accidental exposure occurs, instruct patient to flush eyes with water and seek medical care as soon as possible.
Local Skin Reactions
Local skin reactions, including severe reactions (erythema, flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ulceration) in the treated area can occur after topical application of KLISYRI. Occlusion after topical application of KLISYRI is more likely to result in irritation. Avoid use until skin is healed from any previous drug, procedure, or surgical treatment.
ADVERSE REACTIONS
The most common adverse reactions (incidence ≥2%) were local skin reactions, application site pruritus, and application site pain.
Please see full Prescribing Information.
To report an adverse event or product complaint, call or email Medical Affairs and Customer Relations • Phone: 1-866-665-2782• Fax: 510-595-8183 •
Email: almirallmc@eversana.com
References: 1. KLISYRI®. Prescribing information. Almirall, LLC. 2. Blauvelt A, Kempers S, Lain E, et al. Phase 3 trials of tirbanibulin ointment for actinic keratosis. N Engl J Med. 2021;384:512-520. 3. Blauvelt A, Kempers S, Schlesinger T, et al. Tirbanibulin ointment 1% for actinic keratosis (AK): pooled data from two phase 3 studies. Presented at: 40th Annual Fall Clinical Dermatology Conference (Fall CDC 2020); Virtual Congress; October 29-November 1, 2020. 4. Marson J, Del Rosso J, Bhatia N, et al. Considerations in the management of actinic keratosis: the importance of adherence and persistence to therapy. Skin J Cutan Med. 2021;5(2):83-89. 5. Cerio R. The importance of patient-centred care to overcome barriers in the management of actinic keratosis. JEADV. 2017;31(suppl 2):17-20. 6. Data on file. 2024. Tirbanibulin clinical photos. 7. Data on file. 2021. Pivotal Clinical Trials. 8. Data on file. 2019. KX01-AK-003 9. Data on file. 2019. KX01-AK-004. 10. Smolinski MP, Bu Y, Clements J, et al. Discovery of novel dual mechanism of action Src signaling and tubulin polymerization inhibitors (KX2-391 and KX2-361). J Med Chem. 2018;61(11):4704-4719. 11. Kempers S, DuBois J, Forman S, et al. Tirbanibulin ointment 1% as a novel treatment for actinic keratosis: phase 1 and 2 results. J Drugs Dermatol. 2020;19(11):1093-1100. 12. Niu L, Yang J, Yan W, et al. Reversible binding of the anticancer drug KXO1 (tirbanibulin) to the colchicine-binding site of β-tubulin explains KXO1’s low clinical toxicity. J Biol Chem. 2019;294(48):18099-18108. 13. Efudex®. Prescribing information. Bausch Health US, LLC; 2021. 14. Zyclara®. Prescribing information. Bausch Health US, LLC; 2020. 15. Tolak®. Prescribing information. Hill Dermaceuticals, Inc; 2022.