In the topical field treatment of actinic keratosis on the face or scalp
THE AK LESION-CLEARING EFFICACY YOU EXPECT WITH THE ONLY 5-DAY FACIAL FIELD TREATMENT1
EXPLORE THE EVIDENCEKLISYRI®: Evaluated in the largest clinical trial program of a topical Rx treatment for AK2
KLISYRI® Primary Endpoint
COMPLETE CLEARANCE SEEN WITH A 5-DAY TREATMENT COURSE1,3*
5x more patients achieved complete clearance with KLISYRI® vs vehicle4†
100% AK clearance rates for the two Phase 3 studies for all subjects was 44% (n/N=77/175) KLISYRI® vs 5% (n/N=8/176) vehicle in Study 1, and 54% (n/N=97/178) KLISYRI® vs 13% (n/N=22/73) vehicle in Study 21,3
Primary endpoint results
49% of patients treated with KLISYRI® achieved complete clearance (100% clearance at Day 57) of the face or scalp vs 9% for vehicle1,3
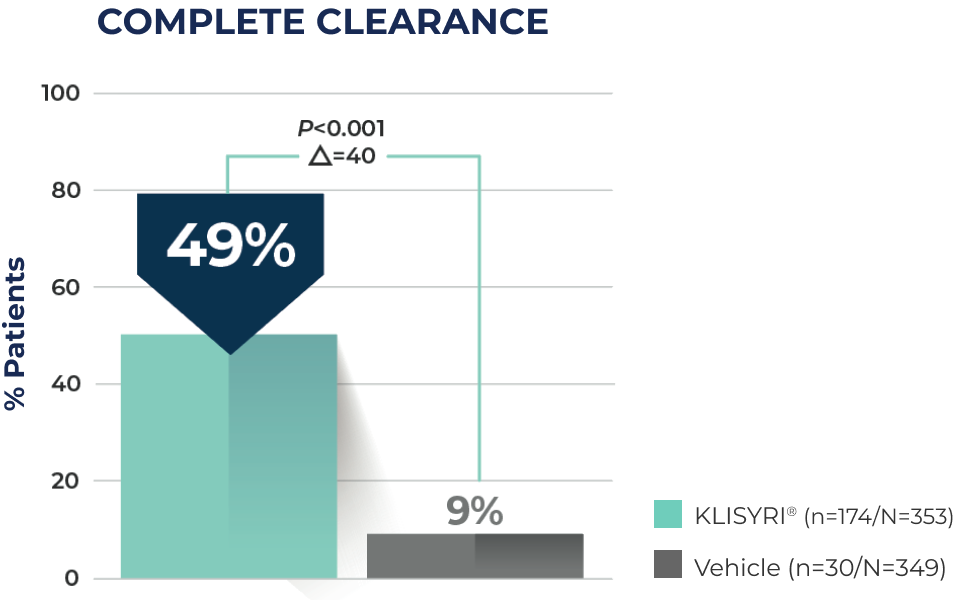
*Complete clearance was defined as a reduction of 100% in the number of lesions in the application area at Day 57.3
†Calculation based on pooled analysis of 702 patients from two Phase 3 studies evaluating all treatment locations (face or scalp): 49% KLISYRI® patients vs 9% for vehicle.1,3,4
Phase 3 Clinical Trials Study Design
KLISYRI®: EVALUATED IN THE LARGEST CLINICAL TRIAL PROGRAM OF A TOPICAL RX TREATMENT FOR AK2
KLISYRI® was studied in two phase 3 clinical trials1,3
Two identical, double-blind, 1:1 randomized, vehicle-controlled clinical trials of 702 patients1
Treatment groups were comparable across all demographics and baseline characteristics, including AK lesion count and distribution on the face or scalp (4-8 clinically typical, visible, and discrete AK lesions in a contiguous 25 cm2 area)1
Primary endpoint: Complete (100%) clearance of AK lesions in the treatment area, defined by the proportion of subjects at Day 57 with no clinically visible AK lesions in the treatment area1
Secondary endpoint: Partial clearance of AK lesions, defined as a reduction of at least 75% in the number of lesions within the application area at Day 571
Face—Before & after results
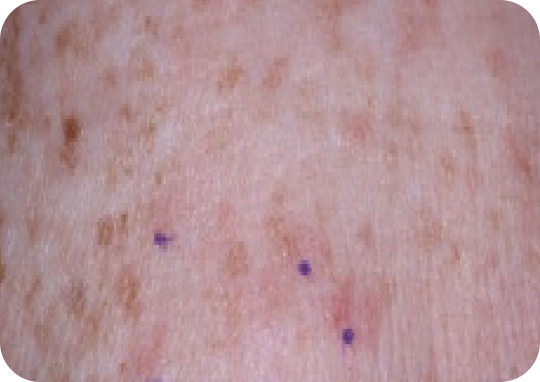
Day 1-Baseline
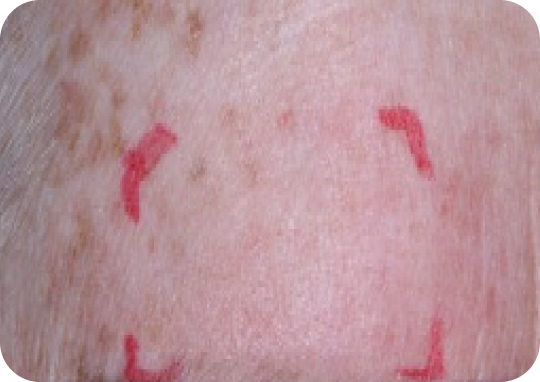
Day 57
Scalp—Before & after results
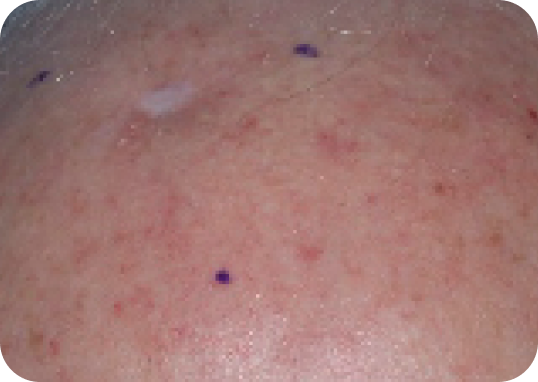
Day 1-Baseline
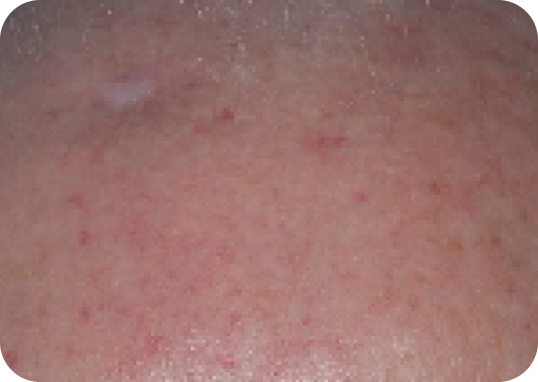
Day 57
Actual clinical trial subject. Results may vary.
Hear from Houston-based,
board-certified dermatologist Stephen Tyring, MD, PhD
—one of the KLISYRI® clinical trial investigators
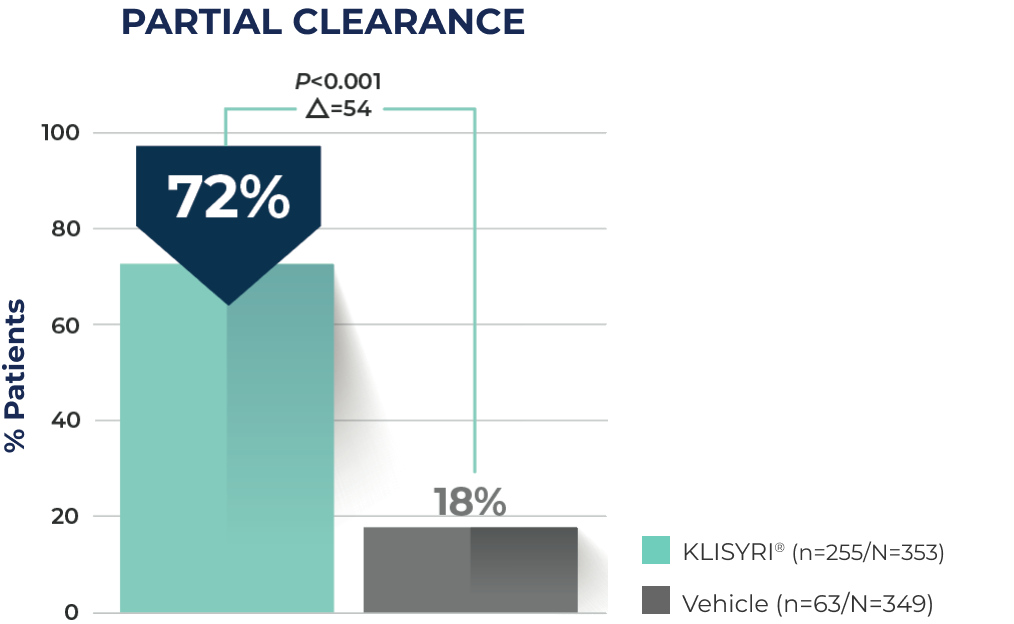
KLISYRI® Secondary Endpoint
PARTIAL CLEARANCE SEEN WITH A 5-DAY TREATMENT COURSE1,3‡
72% of patients treated with KLISYRI® achieved partial lesion clearance (≥75% clearance at Day 57) of the face or scalp vs 18% for vehicle1,3‡§
≥75% AK clearance rates for the two Phase 3 studies for all subjects was 68% (n/N=119/175) KLISYRI® vs 16% (n/N=29/176) vehicle in Study 1, and 76% (n/N=136/178) KLISYRI® vs 20% (n/N=34/173) vehicle in Study 21,3
‡Partial clearance was defined as a reduction of ≥75% in the number of lesions in the treatment area.3
§Calculation based on pooled analysis of 702 patients from two Phase 3 studies evaluating all treatment locations (face or scalp): 72% KLISYRI® patients vs 18% for vehicle.1,3,4
‡Partial clearance was defined as a reduction of ≥75% in the number of lesions in the treatment area.3
§Calculation based on pooled analysis of 702 patients from two Phase 3 studies evaluating all treatment locations (face or scalp): 72% KLISYRI® patients vs 18% for vehicle.1,3,4
Face—Before & after results
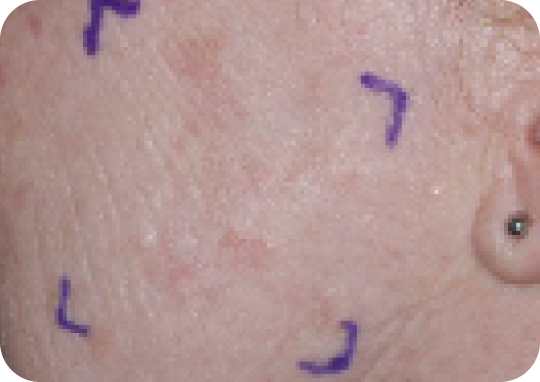
Day 1-Baseline
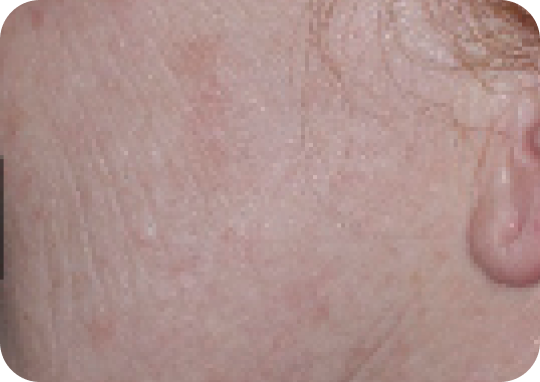
Day 57
Scalp—Before & after results
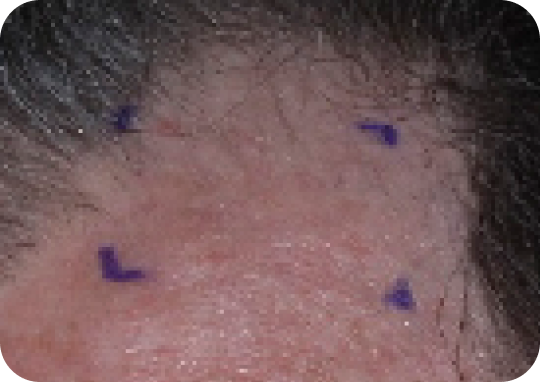
Day 1-Baseline
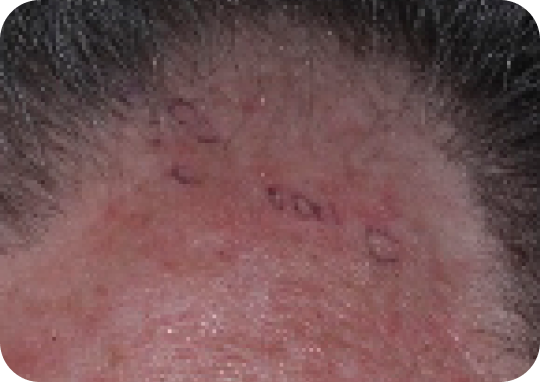
Day 57
Actual clinical trial subject. Results may vary.
SIGNIFICANT REDUCTION OF LESIONS WITH KLISYRI®5-8
Reduction in AK lesion count to Day 57 was significantly greater (P<0.0001) than vehicle for all post baseline visits until Day 575,7,8
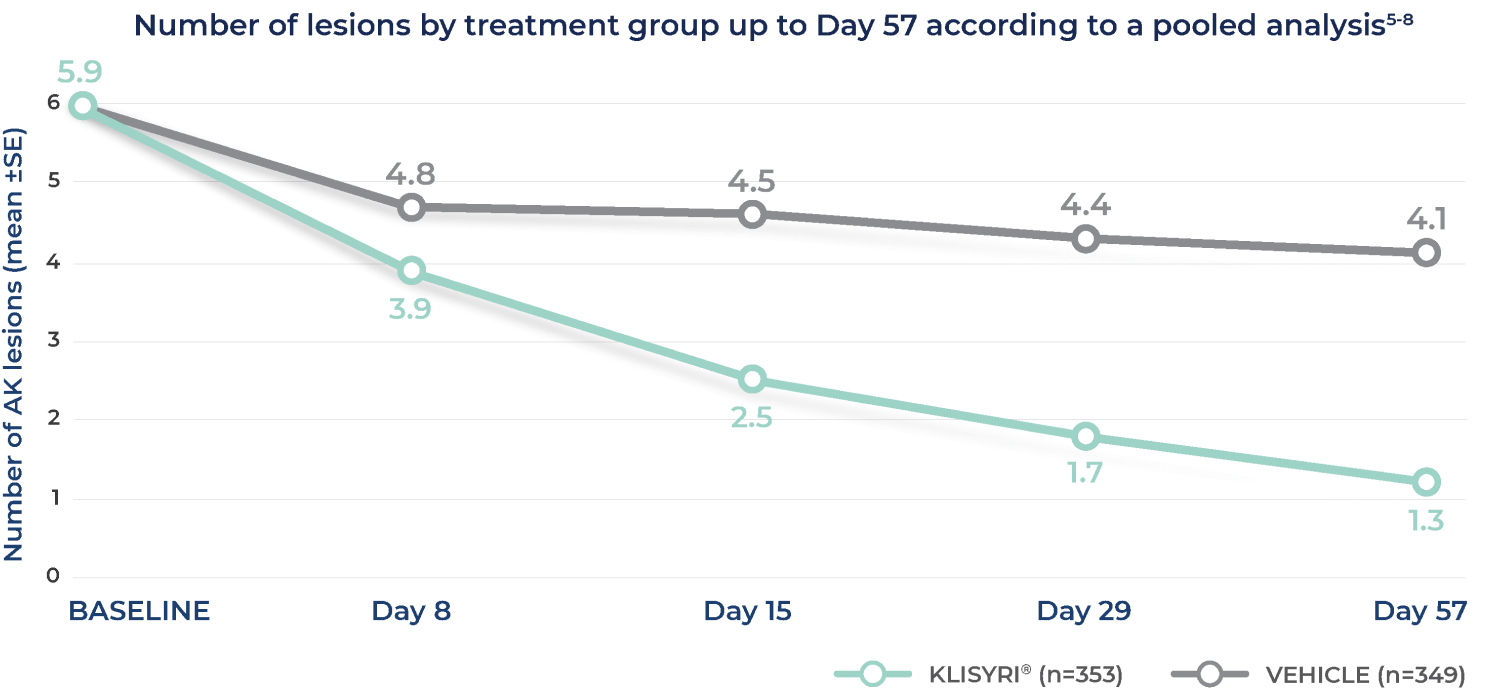
Adapted from Blauvelt A, Kempers S, Lain E, et al. Phase 3 trials of tirbanibulin ointment for actinic keratosis. N Engl J Med. 2021;384(suppl):1-6.
Hear why Philadelphia-based, board-certified dermatologist Mike Stierstorfer, MD, FAAD, started prescribing field therapy with KLISYRI®
Watch videoReal-world evidence at Week 24
IGA success EVALUATED in a real-world study of patients and physicians9,10
Patient- and Clinician-Reported Outcomes for Tirbanibulin in Actinic Keratosis in Clinical Practice Across the United States (PROAK)
PROAK was an observational, single-arm, prospective, multicenter, Phase IV study among 300 adult patients with AK on the face or scalp who initiated treatment with tirbanibulin (per clinical judgment of the Site Investigator) in real-world clinical practices in the US as part of routine care.9
Patients and clinicians completed surveys and clinical assessments concerning the safety and effectiveness of tirbanibulin at baseline, Week 8 (timeframe for primary endpoints), and Week 24 (290 patients were included in the full analyses set).9
The primary endpoint was patient-reported quality of life (QoL) in terms of self-perceived AK symptoms and impact of AK on emotional well-being and functioning, as assessed by the Skindex-16 survey at Week 8. Additional endpoints included (not exclusively) tirbanibulin satisfaction as assessed by the Treatment Satisfaction Questionnaire for Medication (TSQM-9) and the Expert Panel Questionnaire (EPQ) at Week 8, treatment effectiveness assessed by Investigator’s Global Assessment (IGA) at Week 8, investigator rating of severity of photodamage at Weeks 8 and 24, frequency of documented adverse events (AEs), serious adverse events (SAEs), and local skin reactions (LSRs) during the first 8 weeks of the study observation period.
Limitations of the study include the potential for recall bias, reporting bias, selection bias, and other biases commonly seen in real-world and open-label studies. Approaches such as standardized study inclusion/exclusion criteria, consecutive sampling, and geographically diverse dermatology clinics with varied experience with topical field therapy were employed to minimize these biases.
The purpose of the PROAK study was to evaluate patient-reported outcomes (PROs) and clinician-reported outcomes (ClinROs) regarding efficacy and safety among adult patients with AKs on the face or scalp who were administered tirbanibulin in real-world community practices in the US.10
REAL-WORLD EVIDENCE FROM A REAL-WORLD STUDY9,10
Nearly 74% of patients were clear or almost clear at Week 8, and nearly 72% of patients were clear or almost clear at Week 2410
IGA: Investigator’s Global Assessment; W8: Week 8; W24: Week 24.
IGA success was defined as an IGA score of completely cleared (0) or partially cleared (1) in AK status in the treated area at Week 8.
IGA success was a secondary endpoint.
At Week 24, response categories of "don't know/not applicable" were excluded from the analysis.
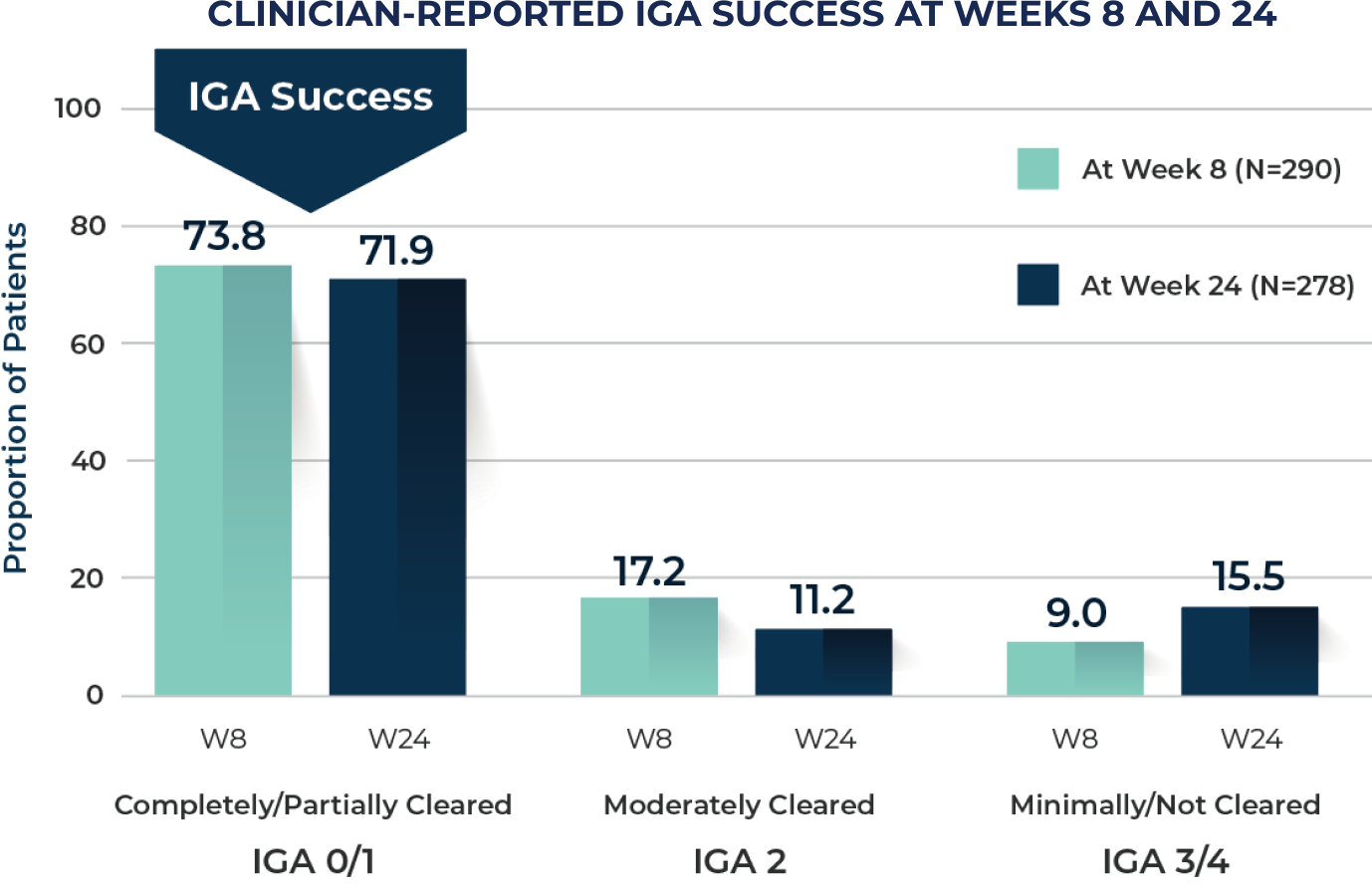
IGA: Investigator’s Global Assessment; W8: Week 8; W24: Week 24.
IGA success was defined as an IGA score of completely cleared (0) or partially cleared (1) in AK status in the treated area at Week 8.
IGA success was a secondary endpoint.
At Week 24, response categories of "don't know/not applicable" were excluded from the analysis.
5-Day Treatment
Subjects received 5 consecutive days of once-daily treatment with either KLISYRI® (n=353) or vehicle control (n=349) to the treatment field1
ADDITIONAL SAFETY STUDY CONFIRMS RESULTS IN A LARGER, 100 cm2 TREATMENT FIELD1¶
AK: actinic keratosis; SE: standard error.
¶25 cm2=3.88 in2; 100 cm2=15.5 in2.
Safety results were comparable to previous controlled studies1
In a separate, multicenter, open-label safety trial, 105 subjects were treated with KLISYRI® once daily for 5 consecutive days in a 100 cm2 treatment field on the face or scalp.1 Subjects enrolled had 4 to 12 clinically typical, visible, and discrete AK lesions. Subjects had an average age of 69 years (range 45 to 87 years),3 were Caucasian (100%), and predominately male (67%) with Fitzpatrick skin types II or III (92%).1
- The safety of KLISYRI® treatment in a 100 cm2 treatment field was comparable to the previous controlled study results in a 25 cm2 treatment field1
IMPORTANT SAFETY INFORMATION
INDICATION
KLISYRI is a microtubule inhibitor indicated for the topical field treatment of actinic keratosis on the face or scalp.
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Ophthalmic Adverse Reactions
KLISYRI may cause eye irritation. Avoid transfer of the drug into the eyes and to the periocular area during and after application. Wash hands immediately after application. If accidental exposure occurs, instruct patient to flush eyes with water and seek medical care as soon as possible.
Local Skin Reactions
Local skin reactions, including severe reactions (erythema, flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ulceration) in the treated area can occur after topical application of KLISYRI. Occlusion after topical application of KLISYRI is more likely to result in irritation. Avoid use until skin is healed from any previous drug, procedure, or surgical treatment.
ADVERSE REACTIONS
The most common adverse reactions (incidence ≥2%) were local skin reactions, application site pruritus, and application site pain.
Please see full Prescribing Information.
To report an adverse event or product complaint, call or email Medical Affairs and Customer Relations • Phone: 1-866-665-2782• Fax: 510-595-8183 •
Email: almirallmc@eversana.com
References: 1. KLISYRI®. Prescribing information. Almirall, LLC. 2. Data on file. 2021. Pivotal Clinical Trials. 3. Blauvelt A, Kempers S, Lain E, et al. Phase 3 trials of tirbanibulin ointment for actinic keratosis. N Engl J Med. 2021;384:512-520. 4. Data on file. 2021. Pooled Data Analysis. 5. Blauvelt A, Kempers S, Schlesinger T, et al. Tirbanibulin ointment 1% for actinic keratosis (AK): pooled data from two phase 3 studies. Presented at: 40th Annual Fall Clinical Dermatology Conference (Fall CDC 2020); Virtual Congress; October 29-November 1, 2020. 6. Blauvelt A, Kempers S, Lain E, et al. Phase 3 trials of tirbanibulin ointment for actinic keratosis. N Engl J Med. 2021;384(suppl):1-6. 7. Data on file. 2019. KX01-AK-003. 8. Data on file. 2019. KX01-AK-004. 9. Data on file 2023. PROAK Study Final Report. 10. Schlesinger T, Kircik L, Lebwohl M, et al. Patient- and clinician-reported outcomes for tirbanibulin in actinic keratosis in clinical practice across the United States (PROAK). J Drugs Dermatol. 2024;23(5):338-346. doi:10.36849/JDD/8264


