Discover 5-day dosing using 350 mg Unit-dose packets1
Discover 5-day dosing using 350 mg Unit-dose packets1
Find out moreA TOPICAL AK TREATMENT WITH shorter duration may help improve patient compliance2
Once daily, 5-day dosing1
A CONVENIENT FACIAL FIELD
TREATMENT FOR YOUR COSMETICALLY
CONSCIOUS PATIENTS1
A CONVENIENT FACIAL FIELD TREATMENT FOR YOUR COSMETICALLY CONSCIOUS PATIENTS1
Complete AK clearance seen with
a short 5-day treatment course1,3
With a convenient, modern dosing period, KLISYRI® is the only 5-day, once-daily topical prescription field treatment for AK—the shortest topical treatment available1,4,5


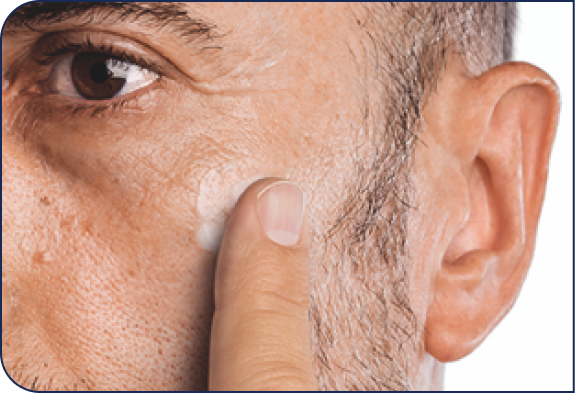
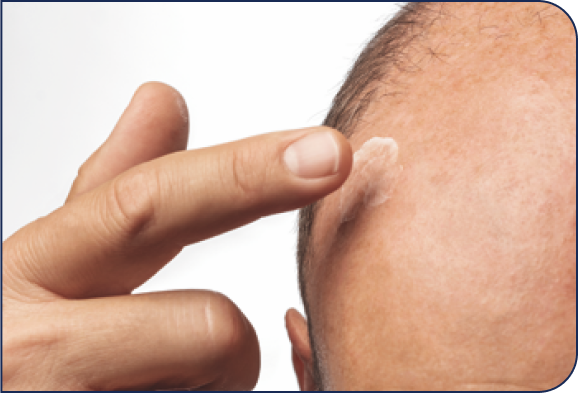
Application Instructions
HELP PATIENTS UNDERSTAND HOW TO APPLY KLISYRI®
Open one packet.

Place ointment
on finger.

Apply evenly in the treatment
area as directed. Patients can
cover up to 100 cm2 with one
pre-measured packet,
depending on packet size.
Patients should wash
hands with soap and
water immediately
after application.

Discard the open packet
after use, even if the entire
packet has not been used.

KLISYRI® may cause eye irritation. Avoid transfer of the drug into the eyes and to the periocular areas during and after application. Wash hands immediately after application.
Avoid washing and touching the treated area for approximately 8 hours after application of KLISYRI®. After 8 hours, you may wash the area with a mild soap and water.1
Hear why Connecticut-based Nurse Practitioner Kimberly Boldon, APRN, likes the convenience of 5-day KLISYRI®
Watch video
High patient compliance
PATIENTS USING KLISYRI® DEMONSTRATED 99% COMPLIANCE IN CLINICAL TRIALS4,5
A modern approach to help your patients with AK get the most from field treatment
KLISYRI® is the shortest topical prescription facial field therapy available for AK—
with patient compliance* as high as 99% in Phase 3 clinical trials4,5
- In an additional single-arm, prospective, real-world cohort study conducted on adults with AK (N=300), 100% of patients completed their 5-day treatment with KLISYRI®6,7
Explore additional treatment compliance
data from a real-world study
*Compliance to the 5-day self-application treatment regimen was >99%.4,5
Convenient field-directed dosing
UNIT-DOSE PACKETS designed to SUPPORT PATIENT COMPLIANCE1,2
Consistent, field-directed dosing
KLISYRI® is available in disposable, pre-measured,
unit-dose packets to ensure consistent field-directed
dosing over just 5 days.1
KLISYRI® is available in disposable, pre-measured, unit-dose packets to ensure consistent field-directed dosing over just 5 days.1
No measuring
No guesswork


See the difference a 5-day treatment can make
SEE BEFORE & AFTER PHOTOSWhat to expect
HELP PATIENTS WITH AK UNDERSTAND WHAT TO EXPECT WITH KLISYRI®
Effective facial field therapy in just 5 treatment days1
Although KLISYRI® is a treatment regimen for only 5 days, topical AK treatments still take time. Be sure to tell your patients that:
- KLISYRI® is the shortest topical Rx treatment for AK1,4,5
Patients should use a total of 5 packets for 5 consecutive days1
If you are giving patients a starter sample, instruct them to use the sample only if they are picking up their full 5-day prescription the very next day.
- Because KLISYRI® prescription packs contain 5 packets of medication, patients who receive a starter sample will have an extra packet and should be told to use 5 packets ONLY
IMPORTANT SAFETY INFORMATION
INDICATION
KLISYRI is a microtubule inhibitor indicated for the topical field treatment of actinic keratosis on the face or scalp.
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Ophthalmic Adverse Reactions
KLISYRI may cause eye irritation. Avoid transfer of the drug into the eyes and to the periocular area during and after application. Wash hands immediately after application. If accidental exposure occurs, instruct patient to flush eyes with water and seek medical care as soon as possible.
Local Skin Reactions
Local skin reactions, including severe reactions (erythema, flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ulceration) in the treated area can occur after topical application of KLISYRI. Occlusion after topical application of KLISYRI is more likely to result in irritation. Avoid use until skin is healed from any previous drug, procedure, or surgical treatment.
ADVERSE REACTIONS
The most common adverse reactions (incidence ≥2%) were local skin reactions, application site pruritus, and application site pain.
Please see full Prescribing Information.
To report an adverse event or product complaint, call or email Medical Affairs and Customer Relations • Phone: 1-866-665-2782• Fax: 510-595-8183 •
Email: almirallmc@eversana.com
References: 1. KLISYRI®. Prescribing information. Almirall, LLC. 2. Shergill B, Zokaie S, Carr AJ. Non-adherence to topical treatments for actinic keratosis. Patient Pref Adherence. 2014;8:35-41. 3. Blauvelt A, Kempers S, Lain E, et al. Phase 3 trials of tirbanibulin ointment for actinic keratosis. N Engl J Med. 2021;384:512-520. 4. Data on file. 2019. KX01-AK-003. 5. Data on file. 2019. KX01-AK-004. 6. Data on file. 2023. PROAK Study Final Report.
7. Schlesinger T, Kircik L, Lebwohl M, et al. Patient- and clinician-reported outcomes for tirbanibulin in actinic keratosis in clinical practice across the United States (PROAK). J Drugs Dermatol. 2024;23(5):338-346. doi:10.36849/JDD/8264
