
You are now leaving www.klisyrihcp.com
You are now leaving KlisyriHCP.com and will be directed to an independent, third-party website. The Privacy Policy and the Terms of Use of the destination website will apply.
Quick Links
KLISYRI is a microtubule inhibitor indicated for the topical treatment of actinic keratosis of the face or scalp
KLISYRI may cause eye irritation. Avoid transfer of the drug into the eyes and to the periocular area during and after application. Wash hands immediately after application. If accidental exposure occurs, instruct patient to flush eyes with water and seek medical care as soon as possible.
Owned by Almirall, LLC.
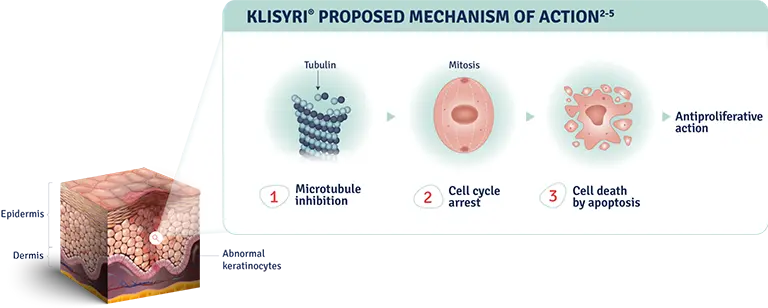
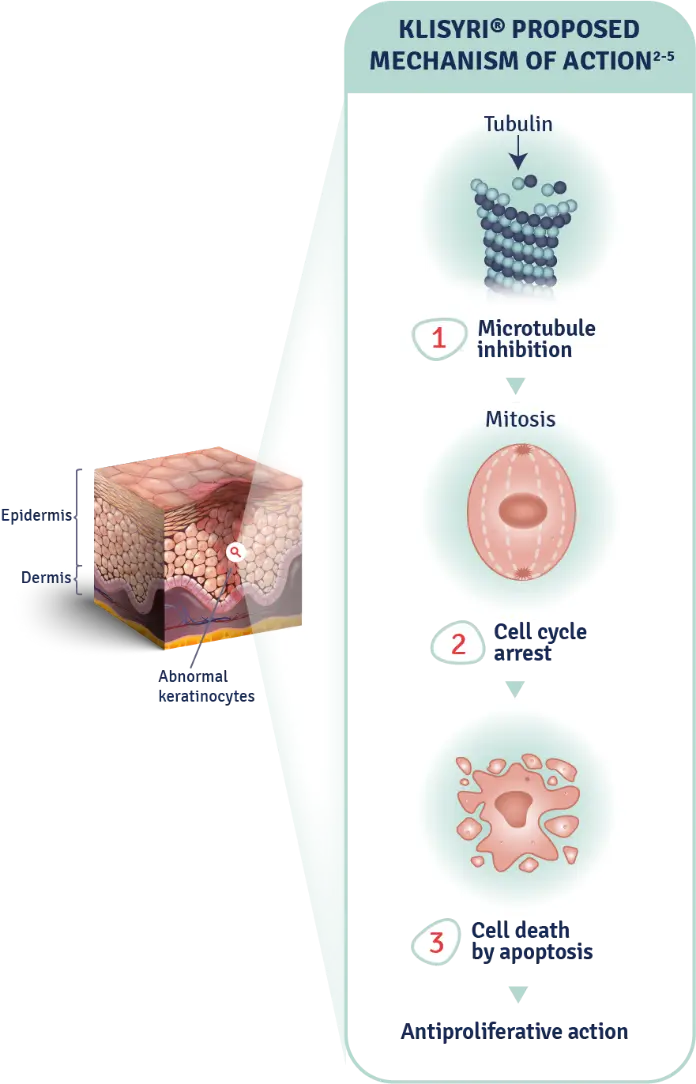
The mechanism of action of KLISYRI® for the topical treatment of actinic keratosis is unknown1
MOA: mechanism of action.
AK: actinic keratosis.
KLISYRI is a microtubule inhibitor indicated for the topical treatment of actinic keratosis of the face or scalp.
KLISYRI may cause eye irritation. Avoid transfer of the drug into the eyes and to the periocular area during and after application. Wash hands immediately after application. If accidental exposure occurs, instruct patient to flush eyes with water and seek medical care as soon as possible.
Local skin reactions, including severe reactions (erythema, flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ulceration) in the treated area can occur after topical application of KLISYRI. Avoid use until skin is healed from any previous drug, procedure, or surgical treatment. Occlusion after topical application of KLISYRI is more likely to result in irritation.
The most common adverse reactions (incidence ≥2%) were local skin reactions, application site pruritus, and application site pain.
To report an adverse event or product complaint, call or email: Medical Affairs and Customer Relations • Phone: 1-866-665-2782 • Fax: 510-595-8183 • Email: almirallmc@eversana.com
KLISYRI is a microtubule inhibitor indicated for the topical treatment of actinic keratosis of the face or scalp.
KLISYRI may cause eye irritation. Avoid transfer of the drug into the eyes and to the periocular area during and after application. Wash hands immediately after application. If accidental exposure occurs, instruct patient to flush eyes with water and seek medical care as soon as possible.
Local skin reactions, including severe reactions (erythema, flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ulceration) in the treated area can occur after topical application of KLISYRI. Avoid use until skin is healed from any previous drug, procedure, or surgical treatment. Occlusion after topical application of KLISYRI is more likely to result in irritation.
The most common adverse reactions (incidence ≥2%) were local skin reactions, application site pruritus, and application site pain.
To report an adverse event or product complaint, call or email: Medical Affairs and Customer Relations • Phone: 1-866-665-2782 • Fax: 510-595-8183 • Email: almirallmc@eversana.com
References:
1. KLISYRI® [package insert]. Malvern, PA: Almirall, LLC, 2021. 2. Smolinski MP, Bu Y, Clements J, et al. Discovery of novel dual mechanism of action Src signaling and tubulin polymerization inhibitors (KX2-391 and KX2-361). J Med Chem. 2018;61(11):4704-4719. 3. Kempers S, DuBois J, Forman S, et al. Tirbanibulin ointment 1% as a novel treatment for actinic keratosis: phase 1 and 2 results. J Drugs Dermatol. 2020;19(11):1093-1100. 4. Niu L, Yang J, Yan W, et al. Reversible binding of the anticancer drug KXO1 (tirbanibulin) to the colchicine-binding site of β-tubulin explains KXO1’s low clinical toxicity. J Biol Chem. 2019;294(48):18099-18108. 5. Yavel R, Overcash JS, Zhi J, Cutler E, Cutler D, Fang J. Phase I maximal use pharmacokinetic study of tirbanibulin ointment 1% in subjects with actinic keratosis. Presented at: 40th Annual Fall Clinical Dermatology Conference (Fall CDC 2020); Virtual Congress; October 29-November 1, 2020.