A MODERN TREATMENT WITH A STRONG RECOMMENDATION FROM THE AMERICAN ACADEMY OF DERMATOLOGY (AAD)1
VIEW AAD RECOMMENDATIONSIn the AAD Work Group’s own words: "The Work Group determined that the overall balance of benefits and potential harms as reported at 57 days favors using tirbanibulin for the management of AK on the face and scalp and that the certainty of the available short-term evidence is high."1
AAD Guidelines of Care
FOR THE FIELD TREATMENT OF AKs, TIRBANIBULIN (KLISYRI®) HAS
A STRONG RECOMMENDATION IN THE AAD’S GUIDELINES OF CARE1
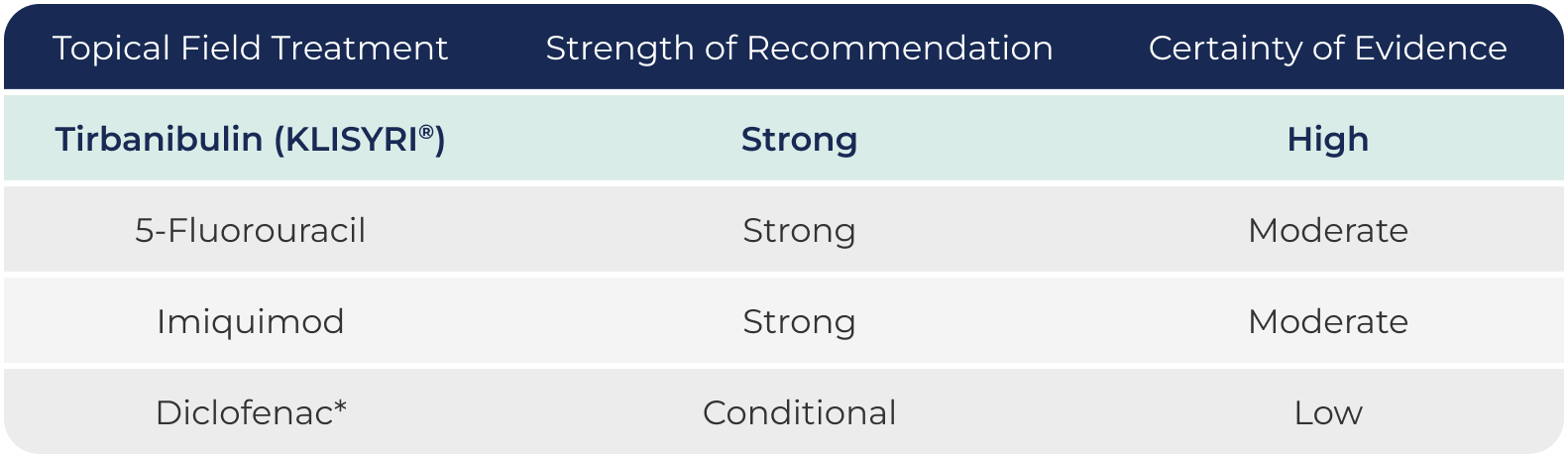
AK Topical
Recommendations From
the Updated Guidelines
of Care1,2
Chart adapted from Focused update to the Guidelines of Care for the Management of Actinic Keratosis: Journal of the American Academy of Dermatology (2022). This chart is not intended to suggest superiority, comparability, or interchangeability among AK treatments.
AK Topical
Recommendations From
the Updated Guidelines
of Care1,2

Chart adapted from Focused update to the Guidelines of Care for the Management of Actinic Keratosis: Journal of the American Academy of Dermatology (2022). This chart is not intended to suggest superiority, comparability, or interchangeability among AK treatments.
AK: actinic keratosis.
*For patients with AKs, nonsteroidal anti-inflammatory drugs (NSAIDs) carry a black box warning for cardiovascular and gastrointestinal side effects.
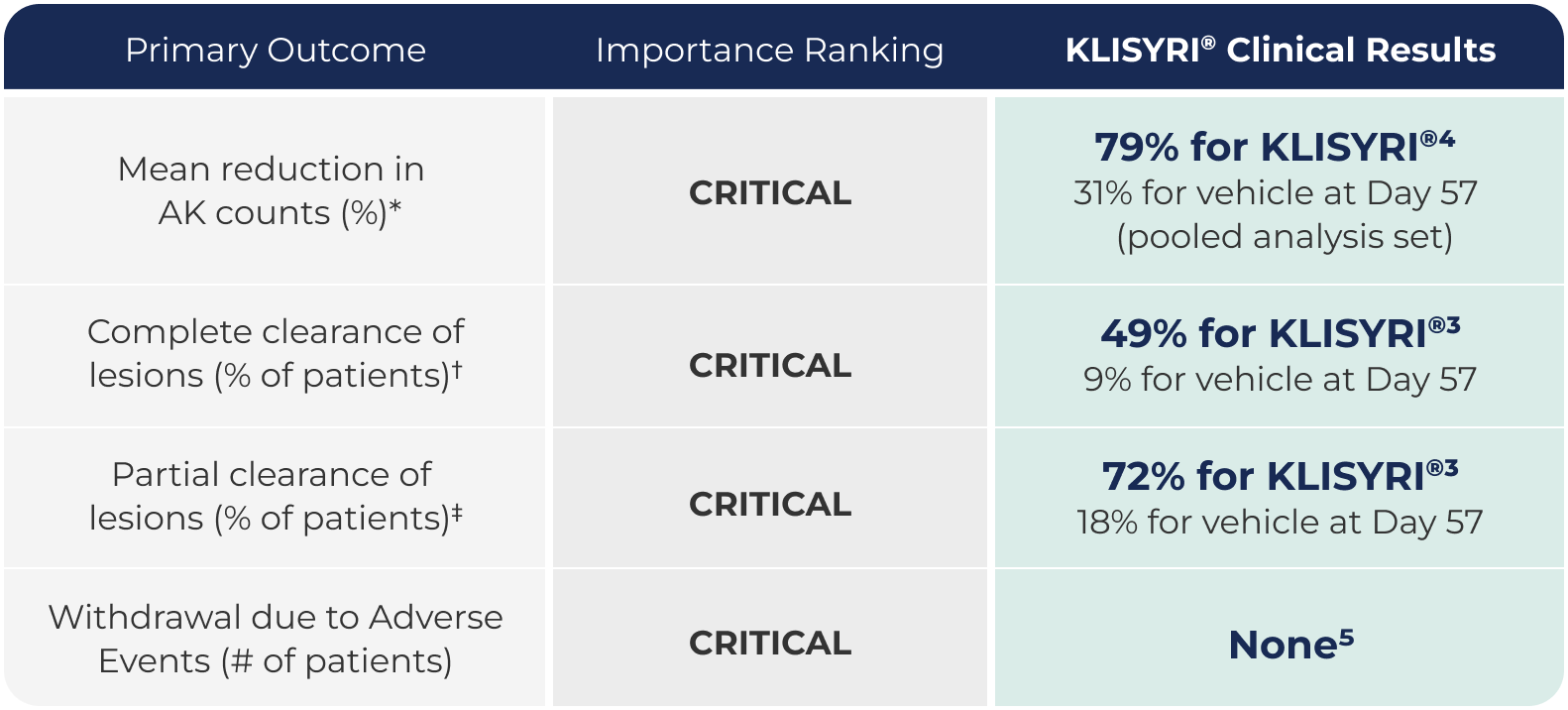
Primary outcome, ranking and results
KLISYRI® (TIRBANIBULIN) SHOWED STRONG CLINICAL OUTCOMES BASED ON THE CRITERIA THAT THE AAD WORKING GROUP RANKED AS CRITICAL1,3
Chart adapted from Focused update to the Guidelines of Care for the Management of Actinic Keratosis: Journal of the American Academy of Dermatology (2022).
*From baseline to assessment.
†Complete clearance of all AKs within a defined field.
‡At least a 75% reduction in AKs in predefined field.
Additional outcomes. Investigator global improvement index (participants rated as "completely improved" by the investigator); Participant global improvement index (participants self-assessed as "completely improved"); and Adverse Events.
IMPORTANT SAFETY INFORMATION
INDICATION
KLISYRI is a microtubule inhibitor indicated for the topical field treatment of actinic keratosis on the face or scalp.
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Ophthalmic Adverse Reactions
KLISYRI may cause eye irritation. Avoid transfer of the drug into the eyes and to the periocular area during and after application. Wash hands immediately after application. If accidental exposure occurs, instruct patient to flush eyes with water and seek medical care as soon as possible.
Local Skin Reactions
Local skin reactions, including severe reactions (erythema, flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ulceration) in the treated area can occur after topical application of KLISYRI. Occlusion after topical application of KLISYRI is more likely to result in irritation. Avoid use until skin is healed from any previous drug, procedure, or surgical treatment.
ADVERSE REACTIONS
The most common adverse reactions (incidence ≥2%) were local skin reactions, application site pruritus, and application site pain.
Please see full Prescribing Information.
To report an adverse event or product complaint, call or email Medical Affairs and Customer Relations • Phone: 1-866-665-2782• Fax: 510-595-8183 •
Email: almirallmc@eversana.com
References: 1. Eisen DB, Dellavalle RP, Frazer-Green L, et al. Focused update: guidelines of care for the management of actinic keratosis. JAAD. 2022;87(2). 2. Eisen DB, Asgari MM, Bennett DD, et al. Guidelines of care for the management of actinic keratosis. JAAD. 2021;85(4):e209-e233. doi: https://doi.org/10.1016/j.jaad.2021.02.082 3. Blauvelt A, Kempers S, Lain E, et al. Phase 3 trials of tirbanibulin ointment for actinic keratosis. N Engl J Med. 2021;384:512-520 4. Blauvelt A, Kempers S, Schlesinger T, et al. Tirbanibulin ointment 1% for actinic keratosis (AK): pooled data from two phase 3 studies. Presented at: 40th Annual Fall Clinical Dermatology Conference (Fall CDC 2020); Virtual Congress; October 29-November 1, 2020. 5. KLISYRI®. Prescribing information. Almirall LLC.